CPV
Home > Products > SphygmoCor >CPV
The SphygmoCor® CPV System
The gold standard in noninvasive central blood pressure and pulse wave velocity assessment
SphygmoCor technology is the gold standard for noninvasive measurement of central blood pressure and pulse wave velocity. It has been featured in hundreds of published clinical studies, and is used in leading medical centers worldwide and in pharmaceutical clinical trials.SphygmoCor systems acquire the patient’s radical pulse wave form through a measurement taken at the wrist, derive the blood pressure wave form at the ascending aorta and report vital central blood pressure data.
The SphygmoCor CPV system also measures pulse wave velocity between two arterial locations.
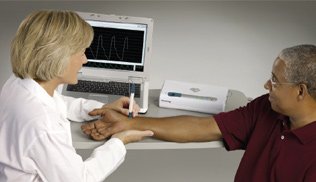
Features
Instantaneous calculation of key parameters, including- Ascending Aortic Blood Pressure
- Pulse Wave Velocity--any two accessible arterial sites
- Aortic Pulse Presssure
- Aortic Augemtentation Index
- Ejection index
- Automatic, real-time QA feedback—minimal potential for operator variability
- Only one operator required
- Noninvasive tests, comfortable for patients
- Heart Rate Variability Option
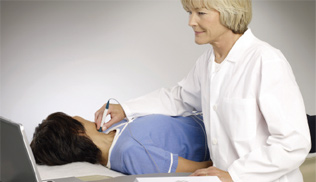
Benefits
- Central pressure proven to be a superior, independent predictor of future cardiovascular events
- Allows assessment of vital drug effects that cannot be detected with brachial pressure measurement
- Provides patients with visual evidence of the effects of lifestyle changes and drug therapies
- Allows integrated analysis of aortic and arterial tree stiffness
SYSTEM SPECIFICATION
Product Configuration
| Product | Inputs |
| SCOR CPV | Tonometer ECG |
| SCOR CPVH | Tonometer ECG |
Physical and Environmental Specifications
| Operating Ambient temperature: | +15°C to 30°C (59°F to 86°F) | |
| Operating Relative humidity | 20% to 80% | |
| Power supply (USB powered): | Supply Voltage Power Consumption Protective Class | USB +5VDC 500 mA Max IEC Class I, II or Internally powered (Depends on Computer that module is connected to. |
| Applied Parts | Type CF (ECG) Type BF (Tonometer) |
|
| Power Connector | Via USB Type A Connector | |
| Physical Specifications | Enclosure Material Weight(Module & Tonometer) Dimensions | PC-ABS 0.8 kg (1.8 lbs) 16.0 (l) x 26.4 (w) x 5.8 (h) cm 6.2” (l) x 10.4” (w) x 2.3” (h) |
Input Signal Specifications
| Input | Specification | |
| Tonometer | Diffused semiconductor whetstone bridge sensor Sensitivity 5 μV/V/mmHg Contact Pressure Range 0 – 300 mmHg Calibration Un-calibrated (Calibrate manually with sphygmomanometer) |
|
| Reference Pressure Bandwidth Sampling Rate Gain & Offset Adjust Signal Range, Accuracy | Atmosphere DC – 40 Hz 128 Hz Auto 10mV, ±5% | |
| ECG | Type Bandwidth | 3-Lead (Lead II) 0.67 – 40 Hz (Device does not support extended low frequency response) |
| Sampling Rate Gain & Offset Adjust Signal Range, Accuracy Heart Rate Range Heart Rate Accuracy |
PWV: 128 Hz HRV: 1024 Hz Auto ±5mV, ±20% 30 BPM to 200 BPM ±10 % |
|
| Footswitch | Type IP Rating |
Micro-switch IPX8-1.0m |
PC Interface Specifications
| Specification | |
| Minimum Computer Requirements. | IBM compatible PC or notebook computer with:
Pentium Processor P4 or greater 1 GB RAM 1024 x 768 256-colour XGA display 60GB initial free hard disc space CD-ROM drive Windows standard printer drivers Dedicated USB port Windows XP Pro or Business Pro only Vista Business or Vista Ultimate only The SphygmoCor® EM3 is not supported on Windows NT/95/98/ME. |
| Unication Interface | USB 1.1 serial interface USB Type B Female connector |
Regulatory
- FDA clearance
- EU CE Mark (MDD, ANNEX II, Class IIa)
- MHLW, Japan
- TGA, Australia
- IEC 60601-1/ AS/NZS 3200.1 (amendments 1 and 2) Electromedical Equipment Safety standard
- IEC 60601-1-2 Electro-Medical Equipment, ElectroMagnetic Compliance (EMC) Standard

